Mastering Marvels of Cellular Energy Chemical Bonds, and Kinetic Potential
CHAPTER 7
Bioenergetics
Introduction:
Understanding the intricate workings of cells forms the cornerstone of modern biological inquiry. Chapter 4 delineated the fundamental structure of cells, elucidating their composition and architecture, while Chapter 6 delved into the pivotal role enzymes play in cellular functions.
A captivating facet of living cells lies in their ceaseless chemical activities, akin to a bustling microcosm where substances enter and exit perpetually. Within these cells, substances undergo breakdown while new compounds emerge, all catalyzed by the omnipresent force of energy.
In the realm of living organisms, energy manifests itself in dual forms: kinetic energy, the dynamic force propelling cellular functions, and potential energy, a reservoir waiting to be tapped for future endeavors. Notably, potential energy lies within chemical bonds, poised to unleash its kinetic counterpart upon their rupture.
This intricate interplay of cellular structure, enzymatic orchestration, and energy dynamics embodies the essence of life’s perpetual motion. The incessant flux of substances in and out of cells, the transformative power of energy, and the delicate equilibrium between potential and kinetic forms weave a narrative of ceaseless activity within the microscopic confines of life itself.
UNDERSTANDING THE CONCEPTS
- Q1. How would you define bioenergetics while relating it to the oxidation-reduction reactions in
living systems?
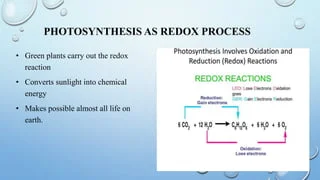
Bioenergetics refers to the study of energy flow in living organisms. It involves understanding how cells convert and use energy from chemical reactions, such as oxidation-reduction reactions, to fuel essential biological processes. Oxidation-reduction reactions are crucial in bioenergetics as they involve the transfer of electrons, which generates energy used by cells to perform various functions.
It delves into the chemical reactions within cells, especially oxidation-reduction reactions. These reactions involve the transfer of electrons between molecules, leading to the release or absorption of energy.
In living systems, oxidation-reduction reactions play a vital role in the production of adenosine triphosphate (ATP), the primary energy currency of cells. During these reactions, molecules change their oxidation states, leading to the release of energy that is captured and stored in ATP molecules for later use by the cell.
Q2. Interpret that ATP is the chief energy currency of all cells.

Adenosine triphosphate (ATP) serves as the primary energy carrier in all cells. It’s essentially the energy currency that powers various cellular processes. When cells need energy for activities like muscle contraction, synthesizing molecules, transporting substances across cell membranes, or powering enzymatic reactions, they rely on ATP.
ATP is composed of an adenosine molecule linked to three phosphate groups. The high-energy bonds between these phosphate groups store potential energy. When ATP is utilized in a cell, it undergoes hydrolysis (a reaction where water breaks a chemical bond), releasing one of its phosphate groups and generating adenosine diphosphate (ADP) along with an inorganic phosphate molecule (Pi). This process liberates energy that the cell can use for various functions.
The significance of ATP lies in its ability to release energy quickly and efficiently. Cells can rapidly regenerate ATP from ADP and Pi through processes like cellular respiration (breaking down food molecules) or photosynthesis (in plants), ensuring a continuous supply of energy for cellular activities.
This constant cycle of ATP synthesis and breakdown allows cells to manage and use energy effectively, making ATP indispensable as the chief energy currency across all living organisms. Interpret that ATP is the chief energy currency of all cells.
Q3. What is the role of chlorophyll and light in photosynthesis?
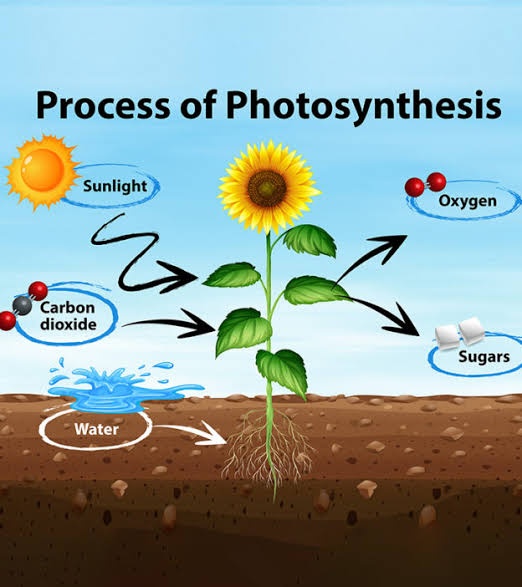
Chlorophyll is like the superhero of photosynthesis! It’s a pigment found in the chloroplasts of plant cells and captures light energy from the sun. This energy is crucial for photosynthesis, the process by which plants make their food. When sunlight hits chlorophyll, it triggers a reaction that splits water molecules and releases oxygen. This process also produces high-energy electrons that kickstart a chain reaction converting carbon dioxide and water into glucose, a type of sugar plants use for energy. So, chlorophyll and light work together to power up photosynthesis, turning sunlight into food for plants.
Q4. Outline the processes involved in photosynthesis.
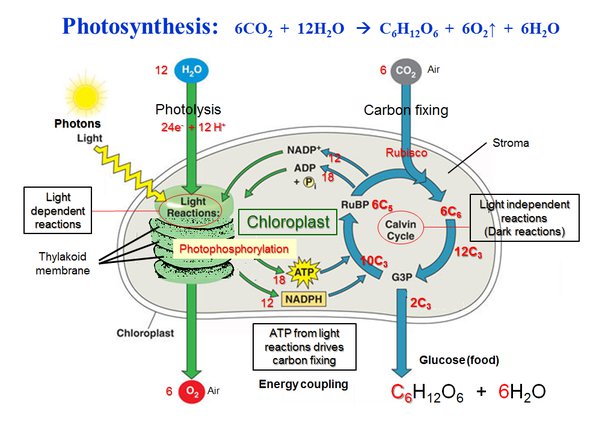
Photosynthesis is a vital process where plants make their food using sunlight, water, and carbon dioxide. It happens in two main stages: the light-dependent reactions and the light-independent reactions (also known as the Calvin cycle).
In the first stage, light-dependent reactions occur in the chloroplasts’ thylakoid membranes. Here, chlorophyll absorbs sunlight, which energizes electrons, splitting water molecules to release oxygen as a byproduct. This process generates ATP and NADPH, which are energy carriers.
The second stage, the Calvin cycle, happens in the chloroplast’s stroma. It uses the ATP and NADPH produced in the first stage along with carbon dioxide to produce glucose. The Calvin cycle involves a series of chemical reactions that convert carbon dioxide into sugars, using the energy from ATP and the high-energy electrons from NADPH.
Overall, photosynthesis is a two-part process where light energy is captured and converted into chemical energy stored in glucose, providing plants with the energy they need to grow and thrive.
Q5. State how the varying light intensity carbon dioxide concentration and temperature affect the
rate of photosynthesis?
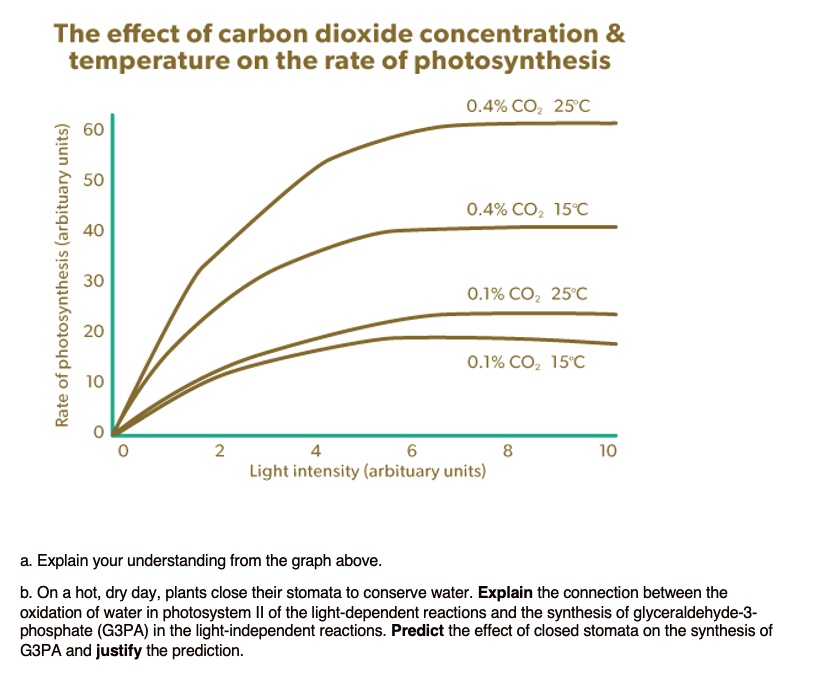
The rate of photosynthesis in plants is influenced by light intensity, carbon dioxide levels, and temperature.
Light intensity: More light means more energy for photosynthesis. Plants can only use a certain amount of light, so after a point, increasing light intensity doesn’t increase the rate of photosynthesis further.
Carbon dioxide concentration: Carbon dioxide is needed for photosynthesis. Higher concentrations of CO2 generally boost the rate of photosynthesis, but like light, there’s a limit to how much plants can use it effectively.
Temperature: Photosynthesis speeds up with higher temperatures, but only to a certain extent. Too high a temperature can slow it down or even damage the plant.
So, optimal conditions for photosynthesis involve a balance: enough light without overwhelming the plant, ample carbon dioxide, and a temperature that’s not too hot or too cold. Finding this balance ensures plants can carry out photosynthesis at their best rate
Q6. Outline the mechanism of respiration while defining glycolysis, Krebs cycle, and electron transport
chain.
respiration is how cells get energy from the food we eat. It happens in three main stages:
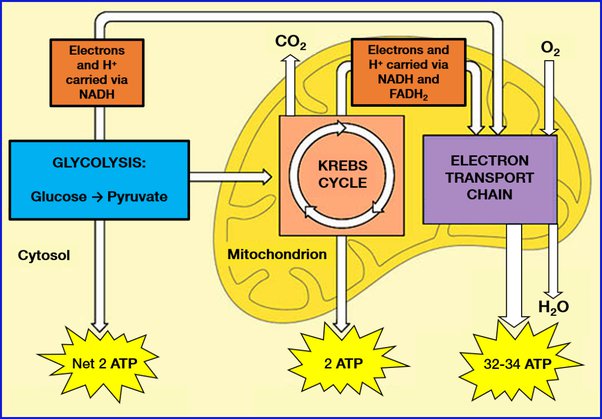
- Glycolysis: This is the first step. It takes place in the cell’s cytoplasm and breaks down glucose (sugar) into smaller molecules, releasing a bit of energy in the form of ATP and some high-energy electrons.
- Krebs Cycle (Citric Acid Cycle): This happens in the mitochondria, the cell’s powerhouses. It’s like the second act. The high-energy electrons produced in glycolysis join with other molecules to produce more ATP through a series of chemical reactions. This cycle also releases carbon dioxide as a waste product.
- Electron Transport Chain: In the mitochondria’s inner membrane, this is where the real energy payoff happens. The high-energy electrons from the Krebs cycle move through a chain of proteins, releasing lots of energy that’s used to make a ton of ATP. At the end of this chain, the electrons combine with oxygen, and water is formed as a byproduct.
Together, these three stages of respiration break down glucose, extract energy from it in the form of ATP, and produce carbon dioxide and water as waste products. This whole process fuels the cells in our bodies, giving them the energy they need to function.
Q7. Draw a comparison of aerobic and anaerobic respiration.

Aerobic and anaerobic respiration are two ways cells produce energy:
- Aerobic Respiration:
- Oxygen Presence: This happens when oxygen is available.
- Efficiency: Produces lots of energy (ATP).
- Products: Generates carbon dioxide and water as byproducts.
- Location: Takes place in mitochondria.
- ATP Yield: Produces more ATP compared to anaerobic respiration.
- Anaerobic Respiration:
- Oxygen Absence: Occurs when oxygen is scarce or unavailable.
- Efficiency: Produces less energy (ATP) compared to aerobic respiration.
- Products: Produces lactic acid or ethanol and carbon dioxide as byproducts.
- Location: Occurs in the cytoplasm of the cell.
- ATP Yield: Yields fewer ATP molecules compared to aerobic respiration.
In summary, aerobic respiration needs oxygen and produces more energy, while anaerobic respiration occurs without oxygen and produces less energy, often resulting in the production of different byproducts like lactic acid or ethanol.
Q8. How will you compare respiration and photosynthesis?
Respiration and photosynthesis are like opposite sides of the same energy coin:
- Respiration:
- Process: Break down food to release energy.
- Purpose: Provides energy for cells.
- Reactants: Uses oxygen and glucose.
- Products: Produces carbon dioxide, water, and ATP.
- Location: Occurs in the mitochondria.
- Photosynthesis:
- Process: Converts light energy into food.
- Purpose: Makes food for plants.
- Reactants: Uses carbon dioxide, water, and sunlight.
- Products: Produces glucose and releases oxygen.
- Location: Takes place in chloroplasts.
In simple terms, while respiration is about breaking down food to produce energy in cells, photosynthesis is about making food (glucose) using sunlight, water, and carbon dioxide, releasing oxygen as a byproduct. They’re like two sides of a cycle where one produces what the other needs to function.
SHORT QUESTIONS
Q1. Why is it said that all life forms are dependent on photosynthesis?
Photosynthesis is the fundamental process that produces food and oxygen in the natural world. It’s the basis of the food chain, as it’s how plants and some other organisms create their food using sunlight, water, and carbon dioxide.
Ultimately, almost all life forms indirectly or directly rely on photosynthesis for their survival. Here’s why:
- Food Production: Photosynthesis creates glucose, which serves as the primary energy source for plants. Herbivores eat these plants for energy, and carnivores eat herbivores. So, all animals, directly or indirectly, depend on plants for food.
- Oxygen Release: Photosynthesis releases oxygen as a byproduct. This oxygen is essential for the survival of most organisms, as they require it for respiration, the process that releases energy from food.
- Ecosystem Balance: Photosynthesis maintains the balance of gases in the atmosphere. Without it, there would be no replenishment of oxygen, and the levels of carbon dioxide in the atmosphere would increase significantly.
In essence, photosynthesis is the cornerstone of life on Earth, as it provides the energy and oxygen necessary for the survival of the majority of organisms in our ecosystem. Without it, life as we know it would not be sustainable.
Q2. What structures and phenomena are involved in the intake of carbon dioxide and water by
plants?
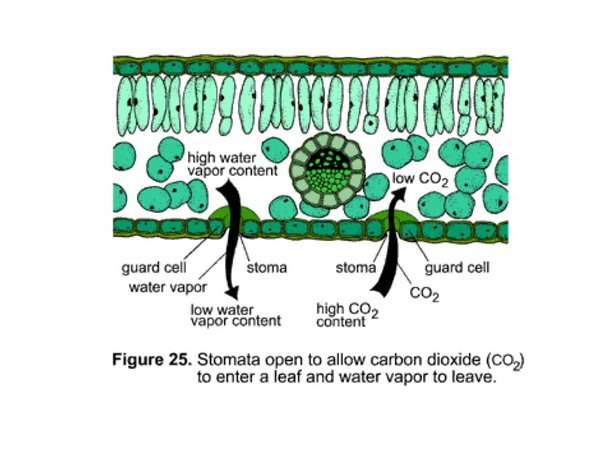
Plants intake carbon dioxide and water through specialized structures and processes:
- Stomata: These are tiny openings on the surface of leaves and stems. They regulate gas exchange, allowing carbon dioxide to enter and oxygen to exit. Stomata open to let in CO2 for photosynthesis and close to prevent excessive water loss.
- Roots: Plants absorb water from the soil through their root systems. Root hairs, tiny extensions on the roots, increase surface area, facilitating water uptake. Water travels from roots to the rest of the plant through specialized tissues called xylem.
- Leaves: The surface of leaves contains cells called mesophyll cells, where most photosynthesis occurs. Within these cells, chloroplasts containing chlorophyll capture light energy used in the photosynthesis process. Carbon dioxide enters the leaves through stomata and dissolves in water within the plant’s cells to start the process of making food.
These structures and processes work together to facilitate the uptake of carbon dioxide and water, which are essential for photosynthesis, the process that plants use to produce food and oxygen.
Q3. In what ways the respiratory energy is used in the body of organisms?
The energy produced during respiration in our bodies is used in various ways:
- Muscle Movement: It’s what powers our muscles for everything from walking to lifting things.
- Cellular Functions: Energy from respiration fuels activities inside our cells, helping them do their jobs effectively.
- Body Maintenance: It’s used to maintain body temperature, repair tissues, and even keep our heart beating steadily.
- Brain Function: Our brain needs this energy to think, process information, and keep everything in our body working smoothly.
- Daily Activities: From digesting food to breathing, every action we take requires this energy from respiration to keep us going throughout the day.
In essence, the energy from respiration is the fuel that keeps our bodies running, allowing us to do everything from the simplest daily tasks to complex thinking and movement.
Q4. What is the importance of anaerobic respiration?
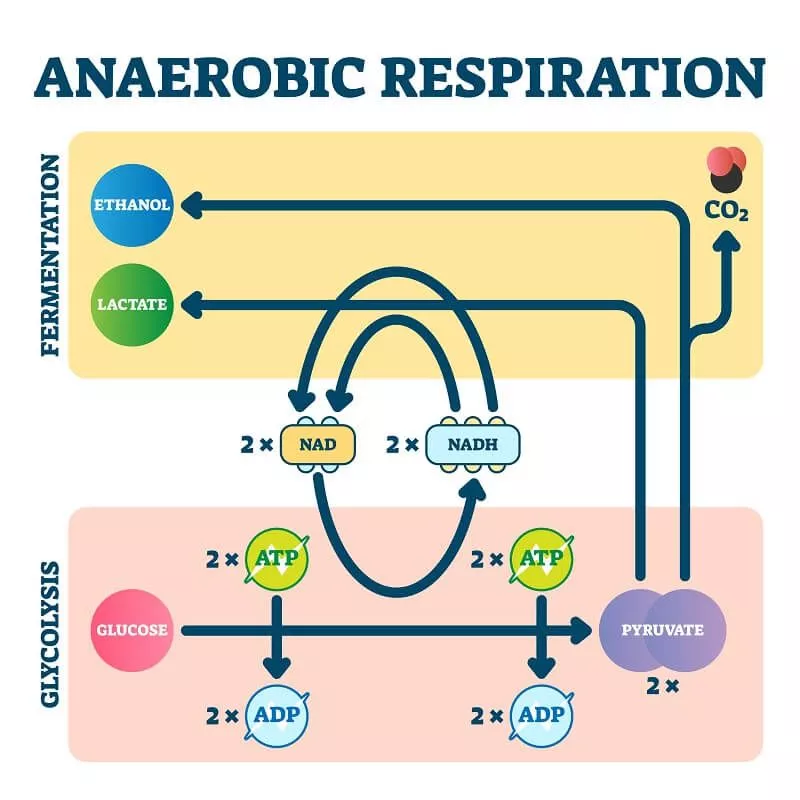
Anaerobic respiration serves crucial purposes:
- Emergency Energy: When oxygen supply is low or unavailable, anaerobic respiration helps generate energy quickly, ensuring cells continue functioning, albeit at a reduced capacity.
- Muscle Power: During intense exercise or when muscles work strenuously, anaerobic respiration kicks in to provide rapid energy, although less efficiently than aerobic respiration.
- Metabolic Flexibility: It allows organisms to adapt to oxygen-deprived environments or situations where oxygen availability fluctuates.
- Organism Survival: Some organisms, like certain bacteria and fungi, rely solely on anaerobic respiration for survival in environments where oxygen is absent.
Overall, while less efficient than aerobic respiration, anaerobic respiration is vital in situations where oxygen is limited, providing a temporary but crucial source of energy for organisms.
In my point of view:
“Embarking on a journey through the microscopic world of cells, one unravels the enigmatic realm of cellular energy. Within these tiny structures lies a symphony of ceaseless chemical activities, where substances enter and exit, and energy reigns supreme. Chapters on cellular structure unveil the intricate architecture, while insights into enzymatic functions shed light on their pivotal role. Delving deeper, the notion of cells as ‘open systems’ becomes apparent, where substances continuously transform, driven by the omnipresent force of energy. This energy, existing in kinetic and potential forms, resides within chemical bonds, releasing its dynamism through oxidation-reduction reactions. Bioenergetics emerges as the crux, delineating the profound connection between cellular functions, redox reactions, and the vibrant dance of kinetic potential.”
Post Comment